![[Steiner e.Lib Icon]](https://wn.rudolfsteinerelib.org/icons/pix/rsa_icon2.gif)
|

|
|
Rudolf Steiner e.Lib
|
|
|
Second Scientific Lecture-Course: Warmth Course
Rudolf Steiner e.Lib Document

|
|
|
Second Scientific Lecture-Course: Warmth Course
Schmidt Number: S-4003
On-line since: 22nd May, 2002
Stuttgart, March 3rd, 1920.
Today in order to press toward the goal of the first of these
lectures, we will consider some of the relations between the being of
heat and the so-called state of aggregation. By this state of
aggregation I mean what I referred to yesterday as called in the
ancient view of the physical world, earth, water, air. You are
acquainted with the fact that earth, water, and air, or as they are
called today, solid, fluid, and gaseous bodies may be transformed one
into another. In this process however, a peculiar phenomenon shows
itself so far as heat relations are concerned. I will first describe
the phenomenon and then we will demonstrate it in a simple fashion. If
we select any solid body and heat it, it will become warmer and warmer
and finally come to a point where it will go over from the solid to
the fluid condition. By means of a thermometer we can determine that
as the body absorbs heat, its temperature rises. At the moment when
the body begins to melt, to become fluid, the thermometer ceases
rising. It remains stationary until the entire body has become fluid,
and only begins to rise again when all of the solid is melted. Thus we
can say: during the process of melting, the thermometer shows no
increase in temperature. It must not be concluded from this however,
that no heat is being absorbed. For if we discontinue heating, the
process of melting will stop. (I will speak more of this
subsequently.) Heat must be added in order to bring about melting, but
the heat does not show itself in the form of an increase in
temperature on the thermometer. The instrument begins to show an
increase in temperature only when the melting has entirely finished,
and the liquid formed from the solid begins to take up the heat. Let
us consider this phenomenon carefully. For you see, this phenomenon
shows discontinuity to exist in the process of temperature rise. We
will collect a number of such facts and these can lead us to a
comprehensive view of heat unless we go over to some reasoned-out
theory. We have prepared here this solid body, sodium thiosulphate,
which solid we will melt. You see here a temperature of about 25° C.
Now we will proceed to heat this body and I will request someone to
come up and watch the temperature to verify the fact that while the
body is melting the temperature does not rise.(Note: The thermometer
went to 48° C. which is the melting point of sodium thiosulphate, and
remained there until the substance had melted.) Now the thermometer
rises rapidly, since the melting is complete, although it remained
stationary during the entire process of melting.
Suppose we illustrate this occurrence in a simple way, as follows: The
temperature rise we will consider as a line sloping upward in this
fashion
(Fig. 1).
Assume we have raised the temperature to the melting
point as it is called. So far as the thermometer shows, the
temperature again rises. It can be shown that through this further
temperature rise, with its corresponding addition of heat, the liquid
in question expands. Now if we heat such a melted body further, the
temperature rises again from the point at which melting took place
(dotted line.) It rises as long as the body remains fluid. We can then
come upon another point at which the liquid begins to boil. Again we
have the same phenomenon as before. The thermometer shows no further
temperature rise until the entire liquid is vaporized. At the moment
when the fluid has vaporized, we would find by holding the thermometer
in the vapor that it again shows a temperature rise (dot-dash line.)
You can see here that during vaporizing the instrument does not rise.
There I find a second place where the thermometer remains stationary.
(Note: the thermometer remained at 100° C. in a vessel of boiling
water.)
Now I will ask you to add to the fact I have brought before you,
another which you will know well from ordinary experience. If you
consider solids, which form our starting point, you know that they
hold their shape of themselves, whatever form is given them they
maintain. If I place a solid here before you it remains as it is. If
you select a fluid, that is, a body that has by the application of
heat been made to go through the melting point, you know that I cannot
handle it piece by piece, but it is necessary to place it in a vessel,
and it takes the form of the vessel, forming a horizontal upper
surface.
(Fig. 3)
If I select a gas — a body that has been
vaporized by passing through the boiling point, I cannot keep it in an
open vessel such as I use for the liquid, it will be lost. Such a gas
or vapor I can hold only in a vessel closed in on all sides, otherwise
the gas spreads out in all directions.
(Fig. 4)
This holds, at least
for superficial observation, and we will consider the matter first in
this way. And now I would ask you to make the following consideration
of these things with me. We make this consideration in order to bring
facts together so that we can reach a general conception of the nature
of heat. Now have we determined the rise in temperature? We have
determined it by means of the expansion of quicksilver. The expansion
has taken place in space. And since at our ordinary temperature
quicksilver is a liquid, we must keep clear in our minds that it is
confined in a vessel, and the three dimensional expansion is summed up
so that we get an expansion in that direction. By reducing the
expansion of quicksilver in three dimensions to a single dimension, we
have made this expansion measure the temperature rise.
Let us proceed from this observation which we have laid out as a
fundamental and consider the following: Assume a line
(Fig. 5)
Naturally, a line can only exist in thought. And suppose on this line
there lie a number of points a, b, c, d, etc. If you wish to
reach these points you can remain in the line. If, for instance, you
are at this point (a) you can reach c by passing along the line.
You can pass back again and again reach the point a. In brief, if I
desire to reach the points a, b, c, d, I can do so and remain
entirely in the line. The matter is otherwise when we consider the
point e or the point f. You cannot remain in the line if you wish
to reach point e or f. You must go outside to reach these points.
You have to move along the line and then out of it to get to these
points.
Now assume you have a surface, let us say the surface of the
blackboard, and again I locate on the surface of this board a number
of points; (a,) (b,) (c,) (d.)
(Fig. 6)
In order to reach these points
you may remain always in the surface of the blackboard. If you are at
this point (x) you may trace your way to each of these points over a
path that does not leave the blackboard. You cannot, however, if you
wish to remain in the surface of the board, reach this point which is
at a distance in front of the board. In this case you must leave the
surface. This consideration leads to a view of the dimensionality of
space from which one can say: To reach points in one dimension,
movement in this single direction suffices, for those in two
dimensions movement in two dimensions gives access to them. It is
however, not possible to reach points outside a single dimension
without leaving this dimension and likewise one cannot pass through
points in three dimensions by moving about in a single plane. What is
involved when I consider the points e and f in relation to the
single dimension represented by points a, b, c, and d? Imagine
a being who was able to observe only one dimension and who had no idea
of a second or third dimension. Such a being would move in his one
dimension just as you do in three dimensional space. If such a being
carried the point a to the position b and the point then slipped
off to e, at that moment the content of the point would simply
vanish from the single dimension of the being. It would no longer
exist for this being from the moment it left the single dimension of
which he is aware. Likewise the points outside a surface would not
exist for a being aware only of two dimensions. When a point dropped
out of the plane, such a being would have no way of following it; the
point would disappear form his space realm. What kind of a geometry
would a unidimensional being have? He would have a one-dimensional
geometry. He would be able to speak only of distance and the like, of
the laws relating to such things as they applied in a single
dimension. A two-dimensional being would be able to speak of the laws
of plane figures and would have a two-dimensional geometry. We men
have at the outset a three-dimensional geometry. A being with a
unidimensional geometry would have no possibility of understanding
what a point does when it leaves the single dimension. A being with a
two-dimensional geometry would be unable to follow the motion of a
point that left a surface and moved out in front of it as we supposed
was the case when the point left a surface and moved out in front of
it as we supposed was the case when the point left the surface of the
blackboard. We men — I state again — have a
three-dimensional geometry. Now I may just as well do what I am
obliged to do on account of the reducing of the three-dimensional
expansion of the quicksilver to a single dimension. I may draw two
lines in two directions so as to form a system of axes, thus giving as in
Fig. 7
an axis of abscissae and an axis of ordinates. At right
angles to the plane of these two, suppose we have a third line which
we will call a space line. (Referring again to the temperature rise
diagram – tr). Just as soon as I come either to the melting
point or the boiling point, at that moment I am not in a position to proceed
with the line
(Fig. 8).
Theoretically or hypothetically there is no
possibility of continuing the line. Let us assume that we can say, the
rise of temperature is represented by this line. We can proceed along
it and still have a point of connection with our ordinary world. But
we do not as a matter of fact have such a point of connection. For
when I draw this temperature curve and come to the melting or boiling
point, I can only continue the curve from the same point
(x, x in
Fig. 8).
I had reached when the body had begun to melt or vaporize. You can
see from this, that in regard to the melting or boiling point, I am in
a position not different from that of the one-dimensional being when a
point moves out of his first dimension into the second dimension, or
of the two-dimensional being when a point disappears for him into the
third dimension. When the point comes back again and starts from the
same place, or as in
Fig. 5
when the point moves out to one side and
returns, then it is necessary to continue the line on in its one
dimension. Considered simply as an observed phenomenon, when the
temperature rise disappears at the melting and boiling point, it is as
though my temperatures curve were broken, and I had to proceed after a
time from the same point. But what is happening to the heat during
this interruption falls outside the realm in which I draw my curve.
Formally speaking, I may say that I can draw this on the space line.
There is, at first considered — note I say at first — an
analogy present between the disappearance of the point a from the
first and into the second dimension and what happens to the
temperature as shown by the thermometer when the instrument stands
still at the melting point and the boiling point.
Now we have to bring another phenomenon in connection with this.
Please note that in this linking together of phenomena we make
progress, not in elaborating some kind of theory, but in bringing
together phenomena so that they naturally illuminate each other. This
is the distinction between the physics of Goethe that simply places
phenomena side by side so that they throw light on each other, and
modern physics which tends to go over into theories, and to add
thought-out elaborations to the facts. For atoms and molecules are
nothing else but fancies added to the facts.
Let us now consider another phenomenon along with this disappearance
of the temperature recorded by the thermometer during the process of
melting. This other phenomenon meets us when we look at yesterday's
formula. This formula was written:
V - Vo (1 + 3αt + 3α2t2 + α3t3)
You remember that I said yesterday you should pay especial attention
to the last two terms. It is especially important for us at this time
to consider
t3,
the third power of the
temperature. Imagine for a moment ordinary space. In this ordinary
space you speak in mathematical terms of length, breadth, and
thickness. These are actually the three dimensions of space. Now when
we warm a rod, as we did yesterday, we can observe the expansion of
this rod. We can also note the temperature of this rod. There is one
thing we cannot bring about. We cannot bring it about that the rod
while it is expanding, does not give off heat to its surroundings,
that it does not stream out or radiate heat. This we cannot prevent.
It is impossible for us to think — note the word — of
a propagation of heat in one dimension. We can indeed think of a space
extension in one dimension as one does in geometry in the case of a
line. But we cannot under any circumstances imagine heat propagated
along a line. When we consider this matter we cannot say that the
propagation of heat is to be thought of as represented in space in
reality by the line that I have drawn here.
(Fig. 1)
This curve does
not express for me the whole process involved in the heat. Something
else is active besides what I can deduce from the curve. And the
activity of this something changes the entire nature and being of what
is shown by this curve, which I am using as a symbol which may be
considered equally well as a purely arithmetical or geometrical fact.
We have, thus, a peculiar situation. When we try to grasp the heat
condition, in so far as the temperature shows this condition, by means
of an ordinary geometrical line, we find it cannot be done. Now this
has another bearing. Imagine for a moment that I have a line. This
line has a certain length:
l
(Fig. 9)
I square this line, and then I can represent this
l2
by a square surface. Assume that I obtain
l3
then I can represent the third power by a cube, a
solid body. But suppose I obtain the fourth power,
l4.
How
can I represent that? I can pass over from the line to the surface,
from the surface to the solid, but what can I do by following this
same method if I wish to represent the fourth power? I cannot do
anything if I remain in our three-dimensional space. The mathematical
consideration shows this. But we have seen that the heat condition in
so far as it is revealed by temperature is not expressible in space
terms. There is something else in it. If there were not, we could
conceive of the heat condition passing along a rod as confined
entirely to the rod. This, however, is impossible. The consequence of
this is that when I really wish to work in this realm, I ought not to
look upon the powers of
‘t’
in the same manner as the powers of a
quantity measured in space. I cannot think about the powers of
‘t’
in
the same way as those of
‘l’
or of any other mere space quantity.
When, for instance, and I will consider this tomorrow hypothetically,
when I have the first power and find it not expressible as a line,
then the second power
t2
cannot be expressed
as a surface and certainly the third power
t3
cannot be expressed as a solid. In purely mathematical space, it is
only after I have obtained the third power that I get outside of
ordinary space, but in this other case I am quite outside of ordinary
space in the case of the second power and the third as well.
Therefore, you must realize that you have to conceive of
t
as different entirely in its nature from space quantities. You must
consider
t
as something already squared, as a second power and
the squared
t
you must think of as of the third power, the cubed
t
as of the fourth power. This takes us out of ordinary
space. Consider now how this gives our formula a very special aspect.
For the last member, which is in this super-space, forces me to go out
of ordinary space. In such a case when I confine myself to reckoning I
must go beyond three dimensional space for the last member of the
formula. There is such a possibility in purely mathematical formulae.
When you observe a triangle and determine that it has three angles,
you are dealing, at the start, with a conceived triangle. Since merely
thinking about it is not enough to satisfy your senses, you draw it,
but the drawing adds nothing to your idea. You have given, the sum of
the angles is 180, or a right-angled triangle — the square of the
hypotenuse equals the sum of the squares of the other two sides. These
things are handled as I now handle the power of
‘t.’
Let us now go back and see what we have established as fact. This is
the way it is done in geometry. It is always true that when I observe
an actual triangle in bridge construction or elsewhere, the abstract
idea verifies itself. What I have thought of in the abstract
‘t‘
has at first a similarity with melting and vaporizing. (We will gradually
get nearer to the essence of the reality.) Melting and vaporizing I
could not express in terms of the three dimensions of space. The only
way I could force them into the curve was to stop and then continue
again. In order to prove the hypothesis that I made for you, it was
necessary, in the case of the third power, the cube of the
temperature, to go outside of three-dimensional space.
You see, I am showing you how we must, as it were, break a path if we
wish to place together those phenomena which simply by being put side
by side illustrate the being of heat and enable us to attain to an
understanding similar to that reached in the preceding course of
lectures on light.
The physicist Crookes approached this subject from entirely different
hypotheses. It is significant that his considerations led him to a
result similar to the one we have arrived at tentatively and whose
validity we will establish in the next lectures. He also concluded the
temperature changes had essentially to do with a kind of fourth
dimension in space. It is important at this time to give attention to
these things because the relativists, with Einstein at their head,
feel obliged when they go outside of three-dimensional space, to
consider time as the fourth dimension. Thus, in the Einstein formulae,
everywhere one finds time as the fourth dimension. Crookes, on the
other hand, considered the gain or loss of heat as the fourth
dimension. So much for this side-light on historical development.
To these phenomena I would ask you now to add what I have formerly
emphasized. I have said: An ordinary solid may be handled and it will
keep its form,
(Fig. 2).
That is, it has a determinate boundary. A
fluid must be poured into a vessel,
(Fig. 3).
It always forms a flat
upper surface and for the rest takes the shape of the vessel. This is
not so for a gas or vaporous body which extends itself in every
direction. In order to hold it, I must put it into a vessel closed on
all sides,
(Fig. 4).
This completely closed vessel gives it its form.
Thus, in the case of a gas, I have a form only when I shut it in a
vessel closed on all sides. The solid body possesses a form simply by
virtue of the fact that it is a solid body. It has a form of itself,
as it were. Considering the fluid as an intermediate condition, we
will note that the solid and gaseous bodies may be described as
opposites. The solid body provides for itself that which I must add to
the gaseous body, namely the completely surrounding boundary.
Now, however, a peculiar thing occurs in the case of a gas. When you
put a gas into a smaller volume
(Fig. 10),
using the same amount of
gas but contracting the walls all around, you must use pressure. You
have to exert pressure. This means nothing else but that you have to
overcome the pressure of the gas. You do it by exerting pressure on
the walls which give form to the gas. We may state the matter thus:
that a gas which has the tendency to spread out in all directions is
held together by the resistance of the bounding walls. This resistance
is there of itself in the case of the solid body. So that, without any
theorizing, but simply keeping in mind the quite obvious facts, I can
define a polaric contrast between a gas and a solid body in the
following way: That which I must add to the gas from the outside is
present of itself in the solid. But now, if you cool the gas, you
can pass back again to the boiling point and get a liquid from the
vapor, and if you cool further to the melting point, you can get the
solid from the liquid. That is to say, you are able by processes
connected with the heat state to bring about a condition such that you
no longer have to build the form from the outside, but the creation of
form takes place of itself from within. Since I have done nothing but
bring about a change in the heat condition, it is self-evident that
form is related in some way to changes in the heat state. In a solid,
something is present which is not present in a gas. If we hold a wall
up against a solid, the solid does not of itself exert pressure
against the wall unless we ourselves bring this about. When, however,
we enclose a gas in a vessel, the gas presses against the solid wall.
You see, we come upon the concept of pressure and have to bring this
creation of pressure into relation with the heat condition. We have to
say to ourselves: it is necessary to find the exact relation between
the form of solid bodies, the diffusing tendency of gases and the
opposition of the boundary walls that oppose this diffusion. When we
know this relation we can hope really to press forward into the
relation between heat and corporeality.

Figure 1
Figure 2
Figure 3

Figure 4
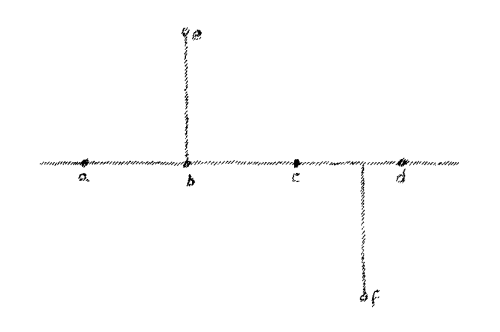
Figure 5
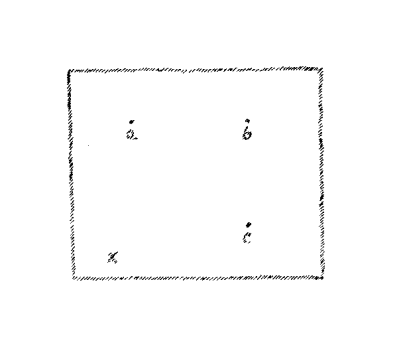
Figure 6

Figure 7
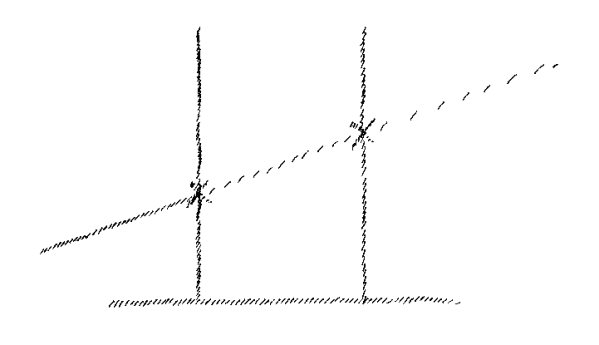
Figure 8
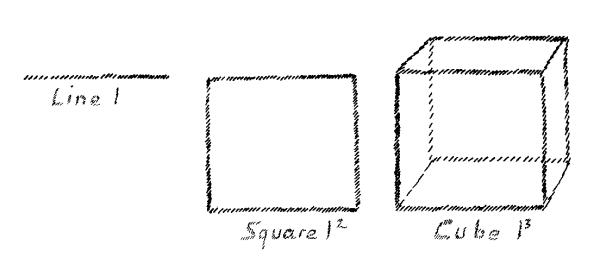
Figure 9

Figure 10
|
|
Last Modified: 04-May-2025
|
The Rudolf Steiner e.Lib is maintained by:
The e.Librarian:
elibrarian@elib.com
|
|
|
![[Spacing]](https://wn.rudolfsteinerelib.org/AIcons/images/space_72.gif)
|

|